| |
| |
Elizabeth Eterigho
Postgraduate Student |
 |
|
| |
|
|
|
| |
Thermocatalytic Cracking of
Natural Feedstocks in Microchannels
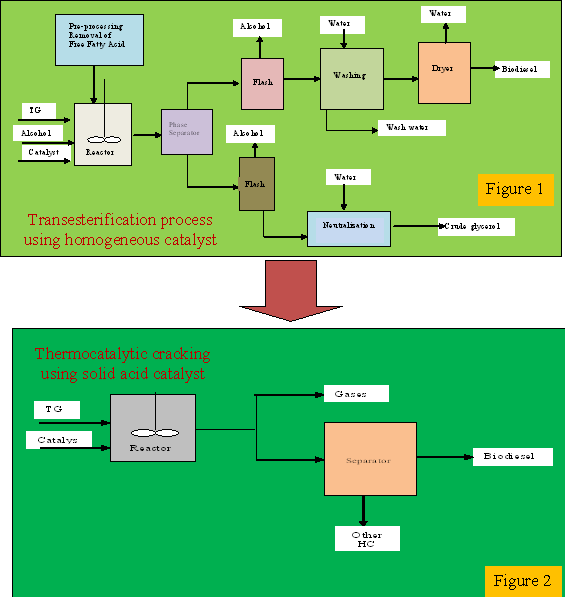
Homogenous catalysed
transesterification is commonly used for
biodiesel production. Although the process has
some advantages such as high conversion rates
and short reaction time, there are a number of
drawbacks: such as soap formation due to free
fatty acids in the feedstock, the catalyst is
not recoverable; however it must be neutralized
for glycerol removal. Even when homogeneous acid
or solid catalysts are used, transesterification
process is far from being environmentally
friendly; this is because the product stream
needs thorough washing. This generates a lot of
waste water which need to be further treated.
Secondly alcohol is still employed in the
process, incurring more cost. Alternatively,
thermocatalytic cracking of triglycerides using
heterogeneous solid-acid catalyst would
eliminate the associated drawbacks caused by
transesterification and reduce the unit
operations as seen from Figures 1 to 2.
 |
 |
Among many heterogeneous
solid-acid catalysts, sulphated zirconia is a
promising catalyst; it is a super-acid catalyst
with H0 =16; that is 104 greater than that of
100 % sulphuric acid, thermally stable and can
be used for organic reaction at low temperatures
compared to zeolites; however its conventional
preparations lead to small surface area and
micro-pores, leaching and high Lewis acid sites
compared to Bronsted acid sites, therefore
restricting its catalytic activity.
Presently, our research has
focused on the improvement of its catalytic
properties (surface area, increased Bronsted
acid sites, and leaching) to produce a high
active and selective catalyst via a solvent-free
method of preparation for enhancing biodiesel
production from triglycerides by thermocatalytic
cracking without the use of alcohol at a
temperature of 270oC. Interestingly the
catalysts were not just active for fatty acid
methyl ester but in addition exhibited some
selectivity for saturated and unsaturated methyl
esters.
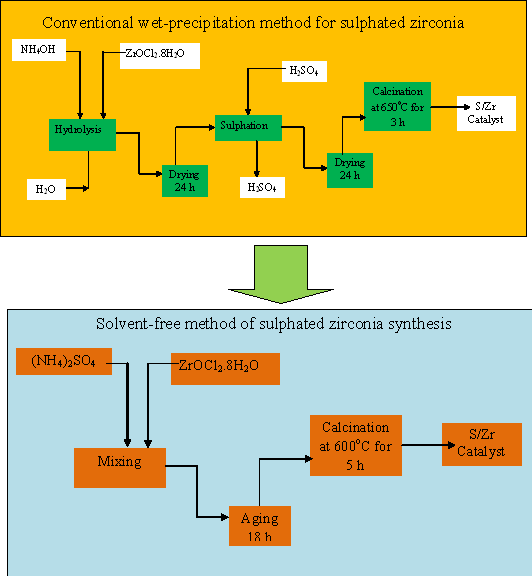
The solvent-free method does
not only enhance the catalytic properties of the
catalyst in addition it is an environmentally
friendly process compared to the conventional
wet-precipitation method of sulphated zirconia
synthesis as shown from Figures 3 to 4. It does
not involve any aqueous medium.
For more information please
contact Prof Adam Harvey
or Dr Jon Lee.
|
|
| |
Publication and Conference
Papers
-
Eterigho, E. J., Lee, J. G.
M. and Harvey, A. P. (2011) 'Triglyceride
cracking for biofuel production using a directly
synthesised sulphated zirconia catalyst',
Bioresource Technology, 102, (10), pp.
6313-6316.
-
Eterigho, E. J., Lee, J. G.
M. and Harvey, A. P. (2011) 'Synthesis,
Characterization and Evaluation of two Forms of
Sulphated Zirconia for Biofuel Production by
Triglyceride Cracking ', Bioenergy III
Conference: Present and New Perspectives on
Biorefineries Lanzarote, Canary Islands, May 22
- 27.
-
Eterigho, E. J., Lee, J. G.
M. and Harvey, A. P. (2011) 'Evaluation of
Catalytic Activity of Synthesized Sulphated
Zirconia for Triglyceride Cracking ', 8th
European Congress of Chemical Engineering.
Berlin, Germany, September 25 - 29.
-
Kasim, F. H., Eterigho, E.
J., Lee, J. G. M. and Harvey, A. P. (2011)
'Catalytic Cracking of Triglyceride by Sulphated
Zirconia for Fatty Acid Methyl Ester with High
Selectivity', 2012 AIChE Annual Meeting.
Minneapolis, MN, United States, October 16-21,
2011.
|
|
|